Maintenance & Organization of Chromatin on the Inactive X Chromosome
The inactive X chromosome (Xi) is the product of the mammalian form of dosage compensation. Dosage compensation serves to balance the levels of X-linked gene expression between the sexes. Early in development, the number of X chromosomes per cell are counted. If there are more than one, then a choice is made as to which X chromosome to silence and which to remain active. For the most part the decision is random, and because this is occurring at a multi-cell stage, the resultant female is a mosaic, with some cells having chosen one X as the Xi while the remaining cells having chosen the other. Inactivation is achieved by repackaging the Xi into facultative heterochromatin. This includes the acquisition of DNA methylation at promoter regions, a variety of histone modifications and substitution of the histone variant macroH2A with core H2A in nucleosomes. In addition, there is a shift in replication timing with the two X chromosomes replicating asynchronously in S-phase. The active X chromosome continues to replicate early in S-phase, whereas the Xi replicates in late S-phase. Combined, these features contribute to silencing of most genes along the length of the X leaving a lone active X chromosome that is equivalent to the single X chromosome found in male cells.
After the choice of which X chromosome to inactivate, that same Xi is stably maintained throughout all subsequent somatic cell divisions. A major interest of the Chadwick lab focuses on how chromatin on the Xi is spatially arranged, variation in the organization, factors involved in maintaining the arrangement and genomic elements that may help promote the organization. the organization and maintenance of chromatin of the inactive X chromosome. The image to the right shows the spatial arrangement of various Xi chromatin markers at metaphase
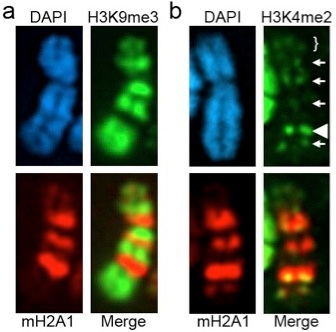
Part (a) shows the distribution of histone H3 tri-methylated at lysine-9 (H3K9me3) relative to the histone variant macroH2A1 (mH2A1). As can be seen, the two do not overlap, but instead occupy discrete alternating territories of the Xi resulting in a banding pattern. Interestingly, the histone modification histone H3 di-methylated at lysine-4 (H3K4me2), a mark typically associated with euchomatin, is observed between the H3K9me3 and mH2A1 bands.
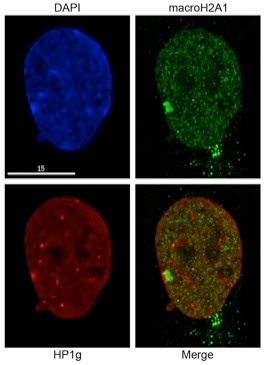
This arrangement is maintained at interphase with the Barr body most often appearing as two adjacent but non-overlapping territories. The image below (left) shows the interphase distribution of mH2A1 and Heterochromatin Protein 1 gamma (HP1g). HP1g is one of three mammalian homologues of drosophila HP1, and binds to methylated lysine-9 of histone H3 through its chromodomain. Therefore it is not surprising that it co-occupies the H3K9me3 territory of the Xi. The same images are shown as a 3-dimensional reconstruction of the nucleus to aid in demonstrating the spatial segregation of the chromatin types.
Role of Macrosatellites in Genome Biology
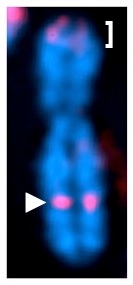
As mentioned above, portions of the Xi are characterized by the euchromatin marker H3K4me2. The most intense of these signals resides approximately mid-way down the long arm of the X chromosome as indicated on the metaphase chromosome to the right by the white arrow-head. H3K4me2 is shown in red. An additional strong signal is detected at the tip of the p-arm (white-bracket). This is not unexpected as this corresponds to the pseudo-autosomal region of the X and is shared with the Y chromosome and therefore not subject to dosage compensation, hence its’ euchromatic appearance. The strong signal on Xq corresponds to the genomic element DXZ4.
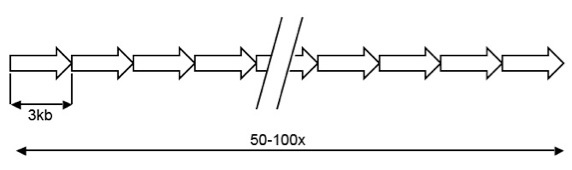
DXZ4 is an X chromosome specific macrosatellite, composed of a 3kb GC rich sequence arranged in tandem for as many as 100 copies, but is highly polymorphic in the general population. A schematic representation of the array is shown below:
The role of macrosatellites in genome biology remains unclear. However, their importance to human health is clearly demonstrated by the Chromosome 4 macrosatellite D4Z4. Like DXZ4, D4Z4 is composed of a 3.3kb GC rich sequence repeated in tandem as many as 120 times, but shares no sequence homology to DXZ4. D4Z4 is located in the subtelomeric region of chromosome 4q35, but is also found as a highly similar array on chromosome 10q26. Strikingly, almost all individuals carrying fewer than 10 copies of D4Z4 at 4q35 develop the third most common form of muscular dystrophy, fascioscapulohumeral muscular dystrophy (FSHD), a disorder characterized by the progressive wasting of muscle in the upper arms and shoulders. How D4Z4 contributes to FSHD remains unclear. Hopefully, our analysis of DXZ4 will hopefully provide useful information to the FSHD community to help determine the molecular basis of the disease.
This research was supported by:

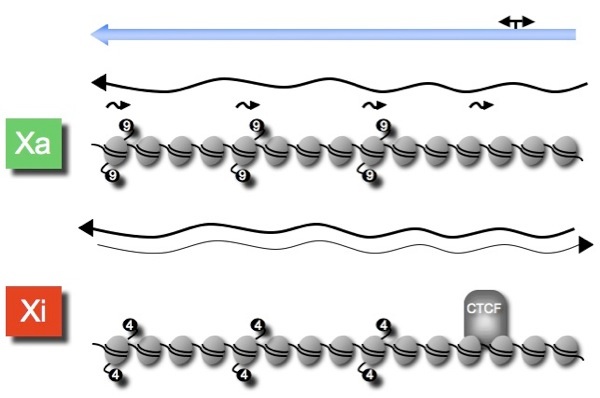
Therefore, DXZ4 adopts an alternate chromatin organization depending upon the environment in which it is embedded: heterochromatic surrounded by euchromatin on the active X and euchromatic surrounded by heterochromatin on the Xi. Furthermore, DXZ4 on the Xi is bound by the epigenetic organizer protein CTCF (See model above). Understanding the role of DXZ4 on the X chromosome is a major goal of the Chadwick lab.
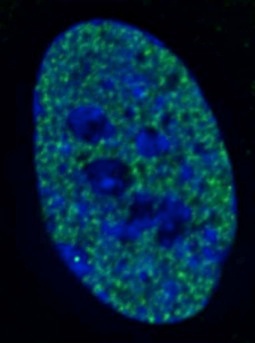
The H3K4me2 signal observed at DXZ4 on the metaphase Xi is clearly visible at interphase. Given that the Xi is largely transcriptionally silent, H3K4me2 is underrepresented at the Barr body. As such, the Barr body appears as a “hole” in the nuclear distribution of H3K4me2, with a bright DXZ4 signal within the hole. An example is shown to the right as indicated by the white arrow.
Interestingly, DXZ4 on the active X and the lone X in males appears to be organized into heterochromatin characterized by H3K9me3. Below is shown a model representing the chromatin organization of a single DXZ4 monomer on the active X and Xi. More details can be found in “Chadwick, (2008) Genome Research, 18:1259-1269