The Nerve Impulse Seen from Outside
Dexter M. Easton July 2000 ©
Next topic Previous topic Table of Contents
Topic 5. The force of diffusion becomes a voltage
The electrical double layer—a result of restrained diffusion—is a useful model for understanding the membrane potential.
The K+ and the Na+ ions tend to diffuse through the specific conductance channels (one type for K+, another for Na+). The channels are highly selective; essentially only K+ can move through K+ channels, only Na+ through Na+ channels. Opposite charges have a strong attraction for one another. Thus, in the axon, the K+ ions are balanced by negative charges on large molecules (P– in Fig. 5) that cannot accompany the K+ ions that are driven to diffuse outward. Similarly, outside, the mainly Cl– ions that balance the many Na+ ions cannot accompany the Na+ ions tending to diffuse inward down the Na+ concentration gradient.
Figure 5. The electrical double layer.
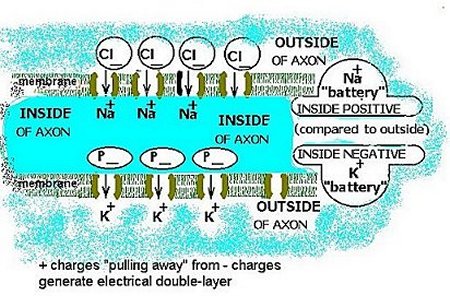
The resolution of this conflict of interests is that the K+ and the Na+ ions penetrate the conductance channels but are restrained from going further by the attraction of the respective counter-ions that cannot penetrate the membrane. Thus the force of diffusion establishes a voltage difference that is the result of an electrical double layer across the membrane. The two participants in this double layer can be viewed as two oppositely directed batteries. The difference in relative concentration (noted in Topic 4) ensures that these batteries do not cancel one another out, and the difference in channel conductance (K+ greater than Na+) ensures that the contribution by K+ is dominant in the resting state of the neurone.
Next topic Previous topic Table of Contents
